
Higher and foundation tiers
The general properties of metals can be summarised in the image below:
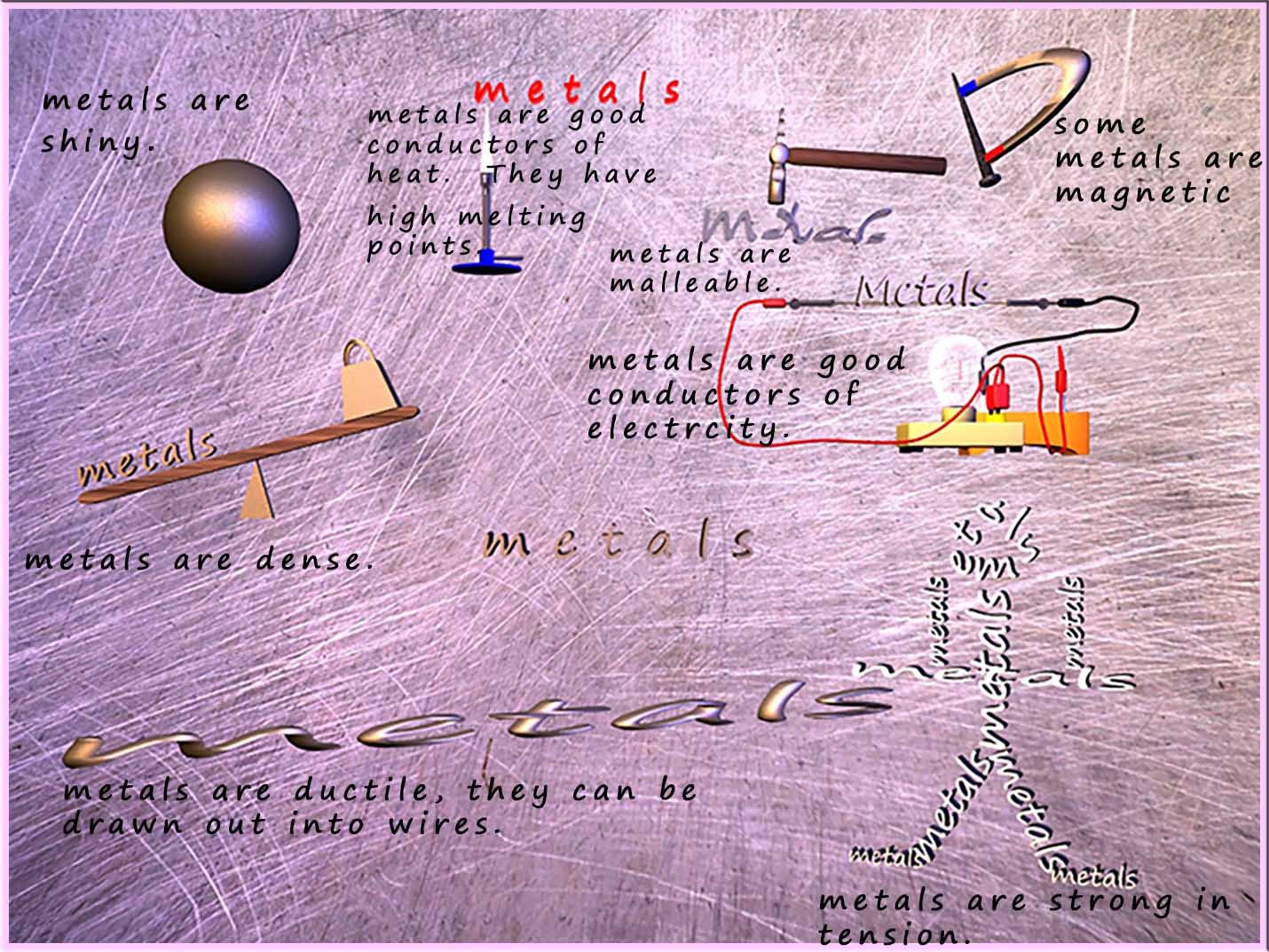
We can use the general properties of metals shown in the image above to help us figure out what the structure of a metal looks like. The table below tries to offer an explanation for some of the properties of metals and relate these properties to a possible feature of the metal structure and the bonding present in the metal.
| Metal property | How this relates to its structure |
|---|---|
| Metals are good conductors of electricity and heat | There must be free or delocalised electrons within the structure to conduct the electricity and heat. |
| Metals have high melting and boiling points. | Metals must have a giant structure with lots of strong bonds. |
| Metals are malleable (can be hammered into shape) and ductile (can be pulled into wire). | There are layers of particles that are able to slide over each other. |
| Metals are shiny. | Free or delocalised electrons are able to reflect light at the surface. |
| Metals are hard and dense | The particles within the metal structure are packed tightly together. |
These basic properties of metals allow us to suggest that
metals consist of a giant structure of ions
which are surrounded by a sea of free moving delocalised electrons.
We also already know that metals are found on the left hand side of the
periodic table in groups 1, 2 and 3 and that
they tend to lose electrons in their reactions to form
positively charged ions.
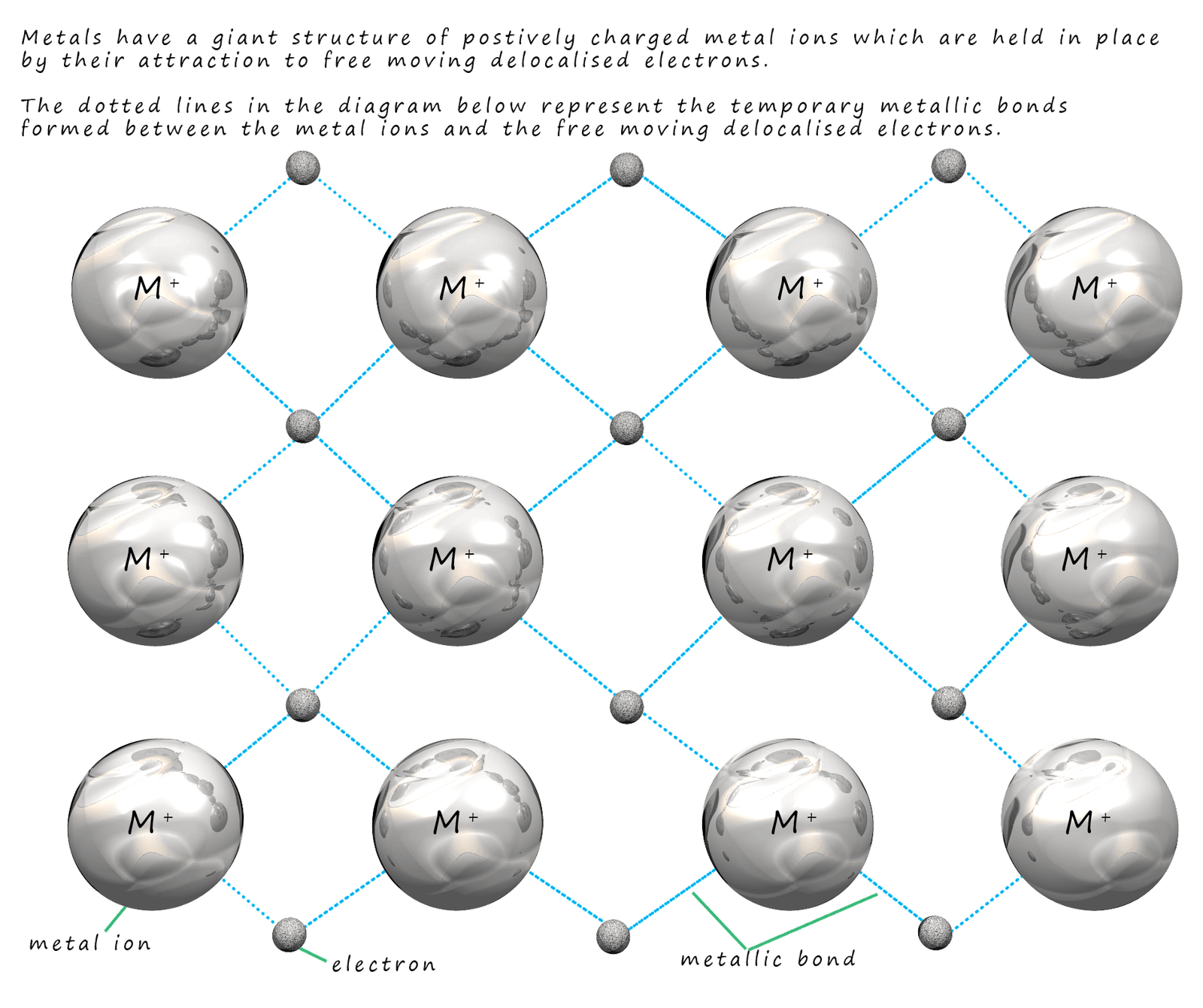
This leads us to the model shown below.
The dark grey balls in the image represent the
positively charged metal ions (atoms which have
lost their outer shell electrons). These
electrons; which are shown as green balls in the image below are delocalised and free to move through the
giant structure of ions.
This might seem odd since we might expect a giant structure of ions which
all have a positive charge to repel each other and so
the structure would simply break down. However we need to think about
the delocalised electrons within the structure and the part they play in holding it together.

The negatively charged delocalised electrons are attracted to the positively charged metal ions and this prevents the metal ions from repelling each other (see image below). The electrons are attracted to the metal ions around them. This attraction of the negatively charged electrons to the neighbouring positively charged metal ions is called a metallic bond. A key feature of this bond is the fact that metallic bonds are not permanent but are constantly breaking and reforming as the electrons move freely around the structure. This is outlined in the diagram below:
The fact that these metallic bonds are not permanent
but are constantly breaking and
reforming allows the layers of ions
to slide over each other. This is shown in the diagram below; which shows
a pushing force being applied to the top two
layers of ions in a metal structure.
The metallic bonds in these layers immediately break
and the layers of ions slide along
but as soon as they stop moving the metallic bonds immediately reform.
This is why metals are malleable (can be hammered or beaten into shape) and ductile (can be pulled into wires).
As mentioned above metals can be hammered and beaten into different shapes; that is they are malleable. The diagram below shows that when a force is applied to layers of metal ions within a giant metal structure the layers of metal ions are able to slide over each other due to the fact that the temporary metallic bonds are continually breaking and reforming. The temporary nature of these metallic bonds also explains why metals can be drawn out into wires; that is they are ductile.

When a force is applied the layers are able to slide over each other. This is possible because the metallic bonds are not permanent but only temporary. When a force is applied the metallic bonds break but instantly reform once the pushing force stops